Greetings everyone!
I am Tibor Bakacs, MD, PhD, DSc, CSO of HepC Inc (https://superinfectiontherapy.com/). Here I summarize our research findings on a paradigm changing antiviral platform technology, the so-called Superinfection Therapy, which could provide a new hope for chronic hepatitis B patients (see references at Blog/News – Superinfection Therapy).
What if the safest antiviral wasn’t synthetic—but a harmless virus itself? We proposed first ‘Fighting Fire with Fire’ – harnessing a naturally occurring, avirulent avian vaccine virus, the Infectious Bursal Disease Virus (IBDV) to fight pathogenic viruses. IBDV could be developed into an off-the-shelf “antibiotic” for viruses. From fighting viruses to empowering the host is a scalable, disruptive approach to antiviral therapy poised for rapid growth.
The problem is that viral diseases remain a top global health threat—with limited treatment options. Most antivirals target the pathogen, making them prone to resistance and narrow spectrum. Pandemic preparedness is constrained by pipeline development time and lack of scalable, rapid-response therapies. Scalable antiviral therapies are urgently needed because the next influenza pandemic is inevitable.
IBDV reprograms the host’s innate immunity, not the virus. IBDV induces host-protective pathways, not inflammation. IBDV has been demonstrated to be safe and effective against five different types of viruses (hepatitis A, B, C viruses, SARS-CoV-2 virus and herpes zoster virus) in more than fifty patients.
New hope for more than 250 million chronic hepatitis B (HBV) patients
As current drugs only suppress but cannot eliminate the virus, we proposed a two-step treatment that mimics how healthy immune systems naturally fight off HBV. The goal is a functional cure—meaning the virus becomes undetectable and stops causing harm, even if some traces remain. First, the immune system should be kickstarted by using the harmless IBDV that triggers a powerful interferon response. This helps shut down HBV replication and reduces the viral load. Then, exhausted immune cells should be rebooted by following up with ultra-low doses of two cancer immunotherapy drugs: nivolumab and ipilimumab. These drugs help “wake up” the body’s HBV-specific T cells so they can finish the job. The immunotherapy combo has been safely used in cancer patients at ultra-low doses.
A doctor, a patient, a breakthrough
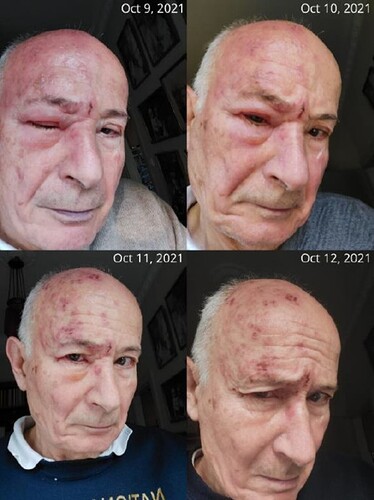
The immunostimulatory power of IBDV superinfection therapy can be best demonstrated by my own severe herpes zoster episode. Imagine waking up one morning with searing pain in your head and around your eye, your vision threatened by escalating infection from the skin into the eye and knowing that conventional treatment might not work fast enough to save it. That was my reality. At 75 years old, I faced herpes zoster ophthalmicus (HZO), a severe form of shingles that attacks the eye. The swelling around my orbit was so intense that I knew I had to act fast (see picture and video).
The CSO of HepC Inc’s HZO with orbital edema at the peak of disease and in recovery. The selfie pictures were taken between October 9, 2021, and October 12, 2021. Consent to the publication of patient information was granted by Tibor Bakacs, M.D., Ph.D., D.Sc., as he was the patient and the treating physician in his autobiography.
Standard antiviral treatment, acyclovir, typically takes two weeks to heal herpes zoster. In older patients, however, as virus-specific host immunity declines with age acyclovir often fails entirely. I had only one chance to protect my eyesight. As both a medical doctor and researcher, I decided to complement acyclovir with the experimental oral IBDV therapy, which is developed by my company. The result? My recovery was astonishing—within just a few days, the painful blisters crusted over, a visible sign of healing that normally takes two weeks on average.