Hello everyone! My name is Daniel Bradley. I am a recent PhD graduate from Dr. John Tavis’s lab. For the past six years, I have been researching the mechanisms of HBV antiviral compounds, studying HBV biology, and helping advance new therapies in preclinical development.
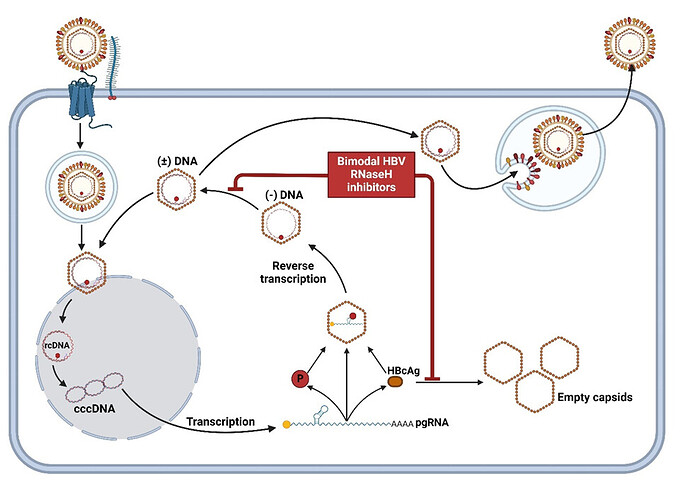
Intro: HBV replicates by reverse transcription. One step during replication is to degrade the pregenomic RNA (pgRNA) after it has been copied into DNA. This is done by the HBV polymerase’s ribonuclease H (RNaseH) activity, and it is essential to make infectious viruses. Inhibiting the RNaseH activity prevents formation of the viral genome and blocks viral replication. The Tavis lab has identified potent inhibitors of the HBV RNaseH and is developing them into potential drugs. We have been researching the effect of HBV RNaseH inhibitors on HBV replication to understand what would occur in people during treatment.
HBV capsids and CAMs: The HBV capsid is a protein shell made of HBV core protein (HBcAg) that assembles around the viral genome. Two basic types of capsids are formed: nucleocapsids (genome-containing) and empty capsids (without the genome). Capsid formation is an essential step for viral replication and is the target for capsid assembly modulators (CAMs). There are two types of CAMs, CAM-A, and CAM-E; both types speed up capsid assembly to the point where they cannot facilitate viral replication.
Results: I found a type of HBV RNaseH inhibitors that unexpectedly decrease capsid levels in two well-studied HBV replication models, HepDES19 and HepG2.2.15 cells. A representative HBV RNaseH inhibitor decreased empty capsid levels without affecting nucleocapsid levels. The inhibitor also decreased capsid accumulation when we expressed only HBcAg in cells, showing that it could affect the capsid independently of its ability to inhibit the RNaseH. Working with Caleb Valkner and Adam Zlotnick to measure capsid assembly in a biochemical reaction, we found that the compound moderately inhibited rather than increased capsid assembly, showing that its effect differed from all known CAMs. We propose that these dual HBV RNaseH-capsid assembly inhibitors are a new class of CAMs called “CAM-I”.
Conclusions: Our research demonstrates that some HBV RNaseH inhibitors have two modes of action (inhibiting RNaseH activity and capsid assembly) (Figure 1). Moving forward, we will determine if bimodal HBV RNaseH-capsid assembly inhibitors can be converted into a new type of HBV drug with two mechanisms of action against HBV.
Figure 1. HBV replication cycle displaying the activities of a bimodal HBV RNaseH inhibitor blocking viral replication and empty capsid formation.