Disclosure: I am the Chief Scientific Officer of and a shareholder in Replicor Inc.
If you have chronic HBV, should you care about HBsAg?
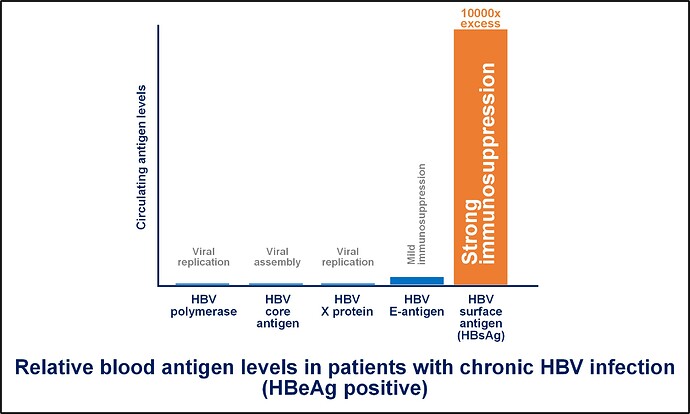
YES! The hepatitis B surface antigen or HBsAg is by far the most abundant viral protein circulating in your blood (at levels 1000’s of times greater than other viral proteins). HBsAg has the ability to suppress several aspects of your immune system’s ability to control your liver infection. Immune control of HBV infection is a very positive outcome which happens for most people who become infected with HBV. In your case, this circulating HBsAg has now made it difficult for your body to control the infection in your liver without medicine (read more about this here).
I have never heard about subviral particles, why are these so important?
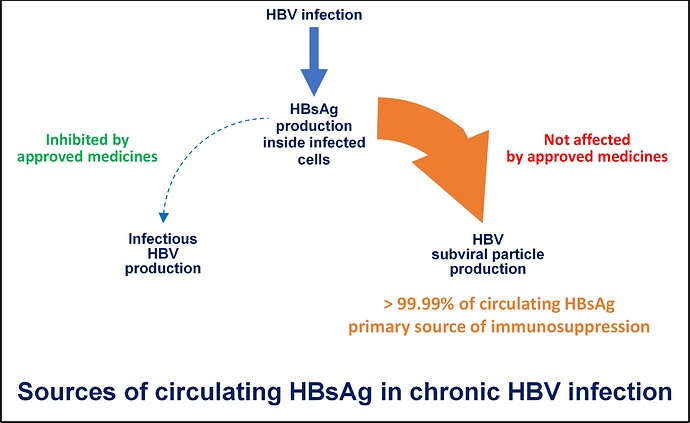
Almost all (more than 99.99%) of the HBsAg in your blood is found in subviral particles. These particles are produced inside infected liver cells by a mechanism that is separate from the process which produces infectious virus. The very large amounts of these subviral particles and the HBsAg they carry are main reason why your HBV infection is chronic. This evolved mechanism of producing “decoy” particles to confound the immune response is also found in many other viral infections but not to the extent found in HBV infection (read more about this here).
Subviral particles have to be removed from your blood to achieve HBsAg loss and to allow your body’s immune response to either recover or to respond productively to immunotherapy. Unfortunately, none of the currently approved treatments for HBV infection (NUCs such as entecavir or tenofovir disoproxil fumarate and or immunotherapy such as pegylated interferon [pegIFN]) interfere with the production of subviral particles and as such, have little impact on your immune system’s ability to control infection. Although these medications work well to control your liver disease, if they are removed, your viral infection will return because the HBsAg-mediated immune suppression remains.
Are there different sources of subviral particles in the liver?
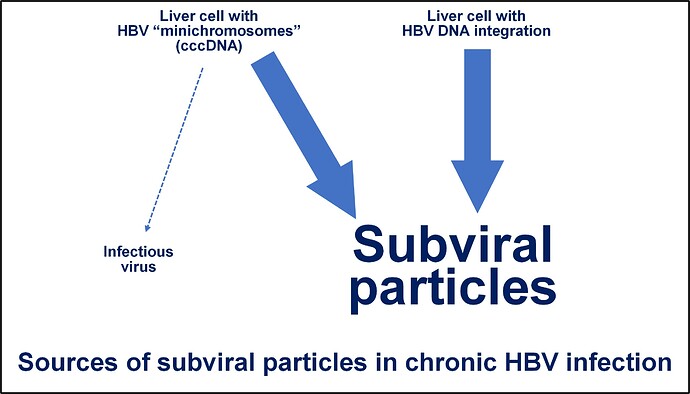
HBV infection establishes two stable sources of HBsAg production. In some liver cells, the genetic template for new viral production is established as a “minichromosome” which behaves like normal chromosomal DNA but is separate from the chromosomes in your cells. In these cells, both infectious virus and subviral particles are produced but the activity of this minichromosome can be controlled by the immune system without their removal. In other liver cells, the genetic template becomes physically inserted into one or more chromosomes. This “integrated HBV DNA” is only able to produce HBsAg so these cells can only produce subviral particles. The extent of HBV DNA integration in the liver gradually increases during the lifetime of HBV infection and this reservoir of subviral particle production can only by removed by immune mediated killing of this cell.
Are there any medications in development which can target subviral particles?
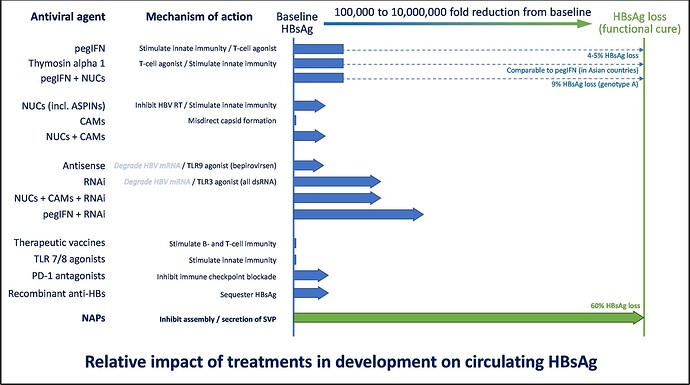
There are several antiviral technologies in development that you may have heard of, including capsid assembly modulators (CAMs), RNA interference (RNAi), antisense oligonucleotides (ASOs) and nucleic acid polymers (NAPs). Of these, only NAPs have been shown to result in the efficient removal of subviral particles in clinical studies of people with chronic HBV infection (read more about this here and here). NAPs are the only drug class which has been able to achieve HBsAg loss during therapy in a majority of patients.
How do nucleic acid polymers work?
Replicor scientists have discovered a protein in hepatocytes which is used in HBV infection to produce subviral particles. This protein is specifically targeted by NAPs, stopping the formation of subviral particles and their release into the blood. In the presence of NAPs, subviral particles are rapidly cleared from the blood by your body, leading to their elimination (read more about this here, here and here).
What about my immune system and “functional cure”?
Functional cure is the term scientists and doctors use to describe completely restored immune control of your chronic HBV infection. This outcome is very similar to the spontaneous control of infection which normally occurs in most people following HBV infection. This is the goal of all current drugs in development for chronic HBV infection: it means someone who is infected no longer has to take medicine – their own immune system effectively controls the infection and allows the liver to heal and functional normally.
For some people, simply clearing HBsAg may be enough to allow their immune system to restore control of their liver infection. However, the longer your chronic HBV infection persists (even while receiving treatment), the more likely your immune system is to become damaged or “put asleep”. As a result, most people will require some form of immunotherapy (while HBsAg is absent) to restore immune function.
How are NAPs being used to achieve functional cure of HBV?
In patients with chronic HBV infection, combination therapy with approved antiviral agents like tenofovir disoproxil fumarate (TDF) and immunotherapy – pegylated interferon alpha 2a (pegIFN) has a very poor activity in the most difficult strain of HBV (genotype D) with 0% of patients achieving functional cure after 48 weeks of therapy. When NAPs are used to clear HBsAg, the functional cure rate in genotype D patients rises to 39%, with an additional 39% of patients with partial cure. This means 78% of patients after receiving 48 weeks of combination therapy with TDF + pegIFN + NAPs no longer need to receive treatment to control their infection. This represents a major breakthrough in the treatment of chronic HBV (read more about this here).
What about HBV co-infection with HDV?
NAPs also directly impact the replication of HDV virus through their interaction with the hepatitis delta antigen, the only HDV viral protein, responsible for replication and assembly of HDV. In a preliminary clinical trial, combination therapy with NAPs and pegIFN was able to eliminate HDV viremia in all patients during therapy with 74% of patients experiencing a “functional cure” of their HDV infection. This means that in the absence of any therapy for at least 3.5 years, no HDV viremia was detected, liver function was normal and liver inflammation and scarring (fibrosis) was regressing. All of these patients also established immune control of their HBV infection (either partial cure or functional cure). Read more about this here, here and here.
What comes next for NAPs?
Replicor’s next trial will use a combination therapy with TDF, immunotherapy and subcutaneously administered REP 2139-Mg (our most advanced NAP drug) in patients with HBV/HDV co-infection to confirm the activity and safety of this treatment in patients with fibrosis and mild cirrhosis. We will compare two different immunotherapies (low dose pegIFN and thymosin alpha 1) to see which one performs better. The results we observe with this combination therapy in HBV / HDV co-infected patients will be applicable to chronic HBV infection in other patients without co-infection or with co-infection with another virus.
What work needs to be completed before NAPs will be available?
REP 2139-Mg is currently in phase IIa trials. Larger phase IIB and phase III trials will be required before regulatory approval can be sought. This process can take anywhere from 3-4 years.
What will NAP treatment look like when it is available?
Therapy will be 48 weeks long. You will take a pill once a day (TDF) and two easy to self-administer injections once each week (immunotherapy and REP 2139-Mg).
Who will benefit from NAP therapy?
NAP-based therapy promises a high rate of functional cure of HBV for the following individuals:
- Any person with chronic HBV regardless of stage of infection, ethnicity, or strain of HBV
- Any person regardless of the extent of liver disease.
- Any person regardless of co-infection (HDV, HIV or HCV).
- A high rate of functional cure of HDV for those with HDV co-infection.